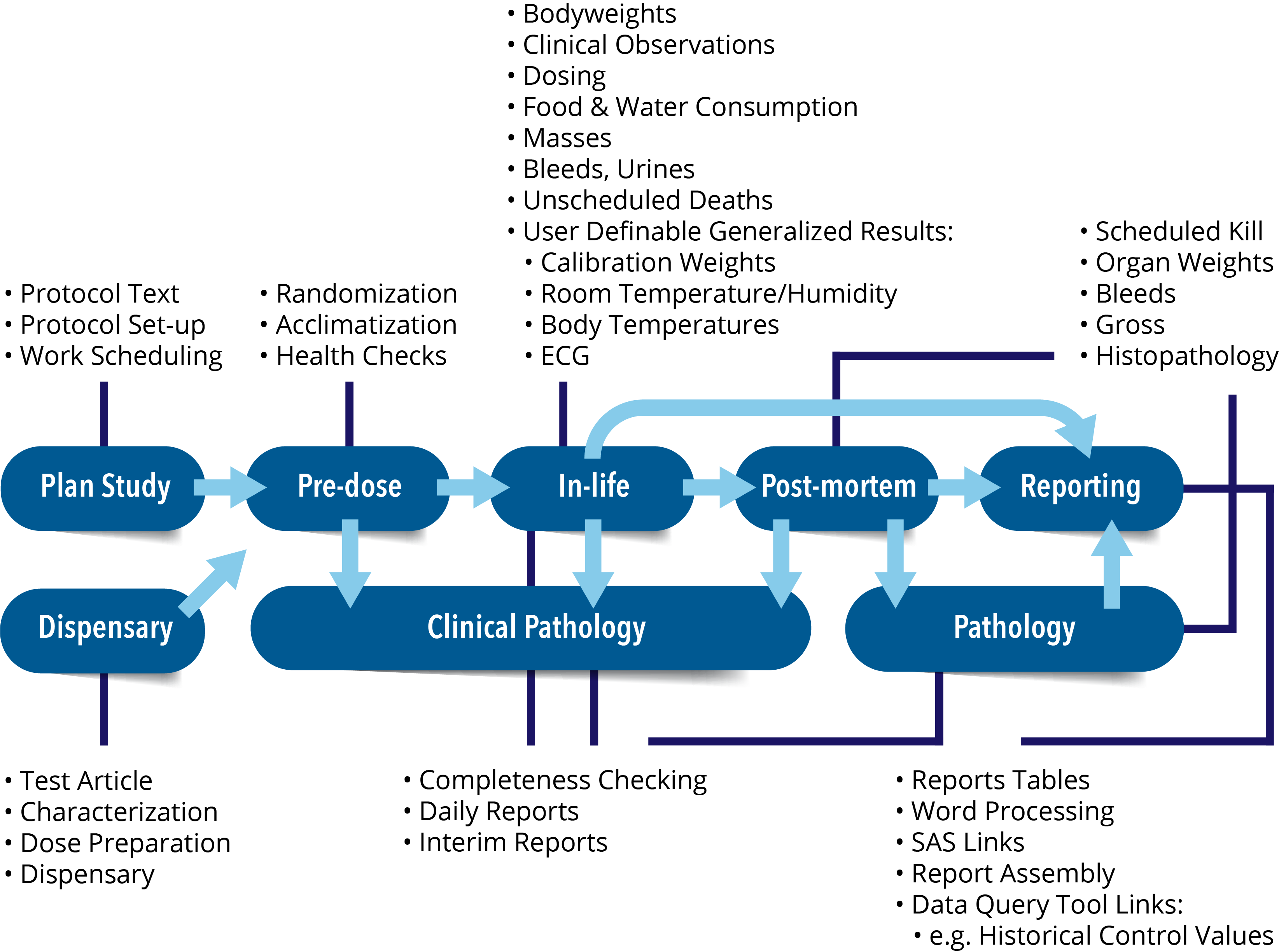
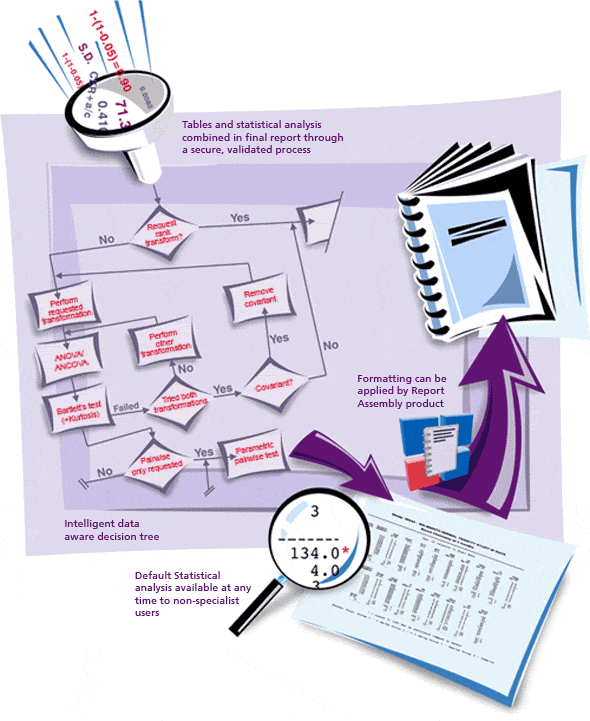
Provantis® is a modern, fully integrated Windows-based system for organizations and universities engaged in non-clinical evaluation studies. From single-user Pathologists to full-function global Toxicology/Pathology laboratories, Provantis streamlines processes and workflows with straightforward, intuitive functionality for simple and complex studies within a GLP or non-GLP environment.
Instem's customer base consists of the leading pharmaceutical, chemical and contract research organizations, including government and privately funded programs across sites worldwide.
The integrated Provantis modules operate in the Microsoft Windows environment, either as traditional desktop-client programs (server-based applications) or through our hosted online offering, allowing customers the ultimate freedom to choose the most appropriate platform for their users.

 On-Demand Education
On-Demand Education
Anywhere, Anytime at a Pace that Suits You!
Enabling Provantis Users to Excel
Provantis users can benefit from the Provantis Academy, an intuitive, easy to use, web-based learning solution that is available on-demand whenever a client needs it. Part of the Instem University eLearning platform, the Provantis Academy curriculum provides users with a personalized approach to learning, giving them access to the training they need anywhere, any time.
Meeting the needs of all users, from super users to staff who only use Provantis infrequently, the Provantis Academy facilitates increased efficiency and effectiveness and fosters a culture of continuous learning.
Provantis Academy users also have direct, live access to Instem’s team of educational industry experts.
All Provantis clients benefit from unlimited access to live global support to ensure operational effectiveness and success. Additionally, Provantis clients can take advantage of our comprehensive Customer Involvement Program (CIP). The CIP offers customers numerous opportunities to engage with Instem staff and fellow users through a variety of forums including our secure, client-only Customer Center website, Value Visits, client webcasts, online & in-person User Group Meetings and Special Interest Group meetings and more.